Welcome to the second entry of The Dianhydrides Diaries, a bi-weekly series that will explore all the benefits of dianhydrides, along with the chemistry, applications, tips, and advice on how to gain a competitive edge with your next project.
Have you ever seen images of space probes, satellites, or space stations and noticed that they are blanketed in what looks like gold foil? If so, did you know that this blanket owes its golden color not to the precious metal it resembles, but to the highly engineered polyimides with which they are constructed? The blanket material is more appropriately called multilayer insulation (MLI) and is wrapped over sections of satellites and spacecraft in orbit which require protection from the solar and electromagnetic radiation in space. It also prevents the loss of heat from the inhabited sections of spacecraft. Note that radiation is the primary mode of heat transfer in and out within the space environment, due to limited air convection in the microgravity of spacecraft. The use of MLI is vital to the uninterrupted operation of the sensitive electronics as well as to the safety of astronauts aboard.
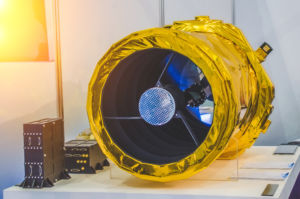
Figure 1. Multilayer insulation (MLI) based on aluminized polyimide films
The polyimide film substrate component central to MLI can withstand temperatures ranging from −260 °C to 400 °C, with short-term exposures of over 480 °C, has low outgassing (making it suitable for use in the vacuum of space), and is resistant to degradation from ultraviolet radiation. The lightweight MLI thus becomes a perfect insulation material for use in space applications. Properly designed MLI is also used as a first line of defense for the spacecraft against dust impacts in space.
MLI is generally produced by metallizing a thin layer of a durable polyimide film, typically via vapor deposition of a thin layer of aluminum onto it. Although the final MLI is still quite thin, it is composed of a collection of even thinner individual layers, hence the term multilayer. The polyimide film central to the construction of the MLI is produced from the reaction of a dianhydride with a diamine in a two-step process (Figure 2), with the resulting polymer having exceptional heat resistance and chemical resistance. Polyimides have glass transition temperatures in range of 300 °C to 400 °C or higher. They also have very high thresholds for thermal or thermo-oxidative degradation.
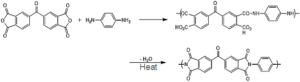
Figure2. The two-step process for making thin polyimide films using a dianhydride and a diamine.
Polyimides with different performance characteristics can be produced by via an appropriate selection of diamine and dianhydride. Commonly used dianhydrides for high temperature performance are based on the aromatic structures, e.g., Benzophenone tetracarboxylic dianhydride (BTDA), Pyromellitic dianhydride (PMDA), Biphenyl tetracarboxylic dianhydride (BPDA), etc.
Now every time you look at images of spacecraft with gold-colored coverings, you can be thankful for the wonderful molecules we call dianhydrides which go into producing this unique and efficient thermal management component called the MLI.
Reference:
1. https://www.nesdis.noaa.gov/content/good-gold-are-satellites-covered-gold-foil


